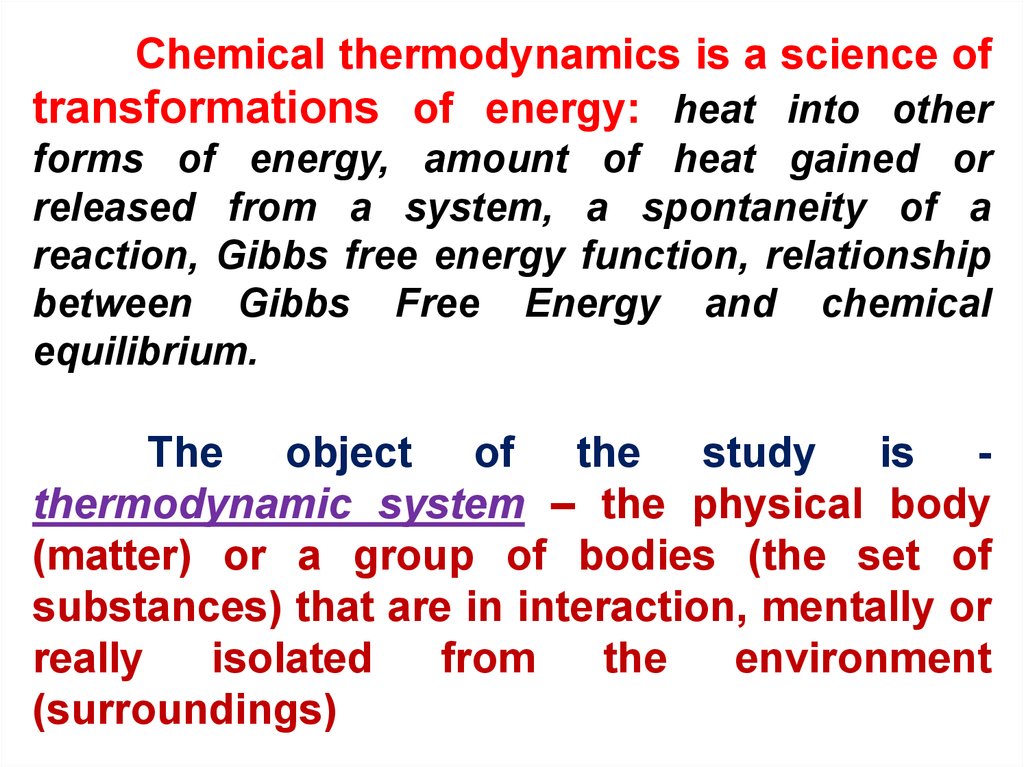
What Is The Change In Free Energy Of A System At Chemical Equilibrium?
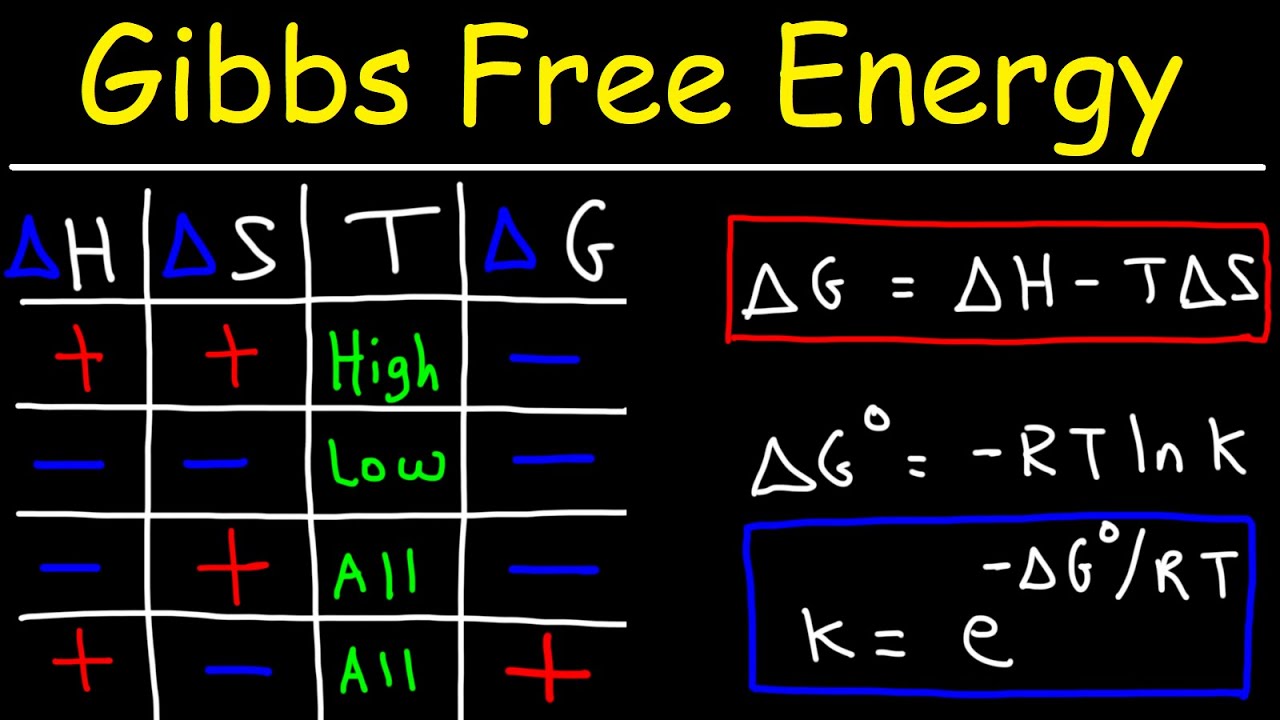
What is the change in free energy of a system at chemical equilibrium?. The unit of measurement in the International System of Units SI of energy is the joule which. The change in the Gibbs free energy of the system that occurs during a reaction is therefore equal to the change in the enthalpy of the system minus the change in the product of the temperature times the entropy of the system. G H - TS If the reaction is run at constant temperature this equation can be written as follows.
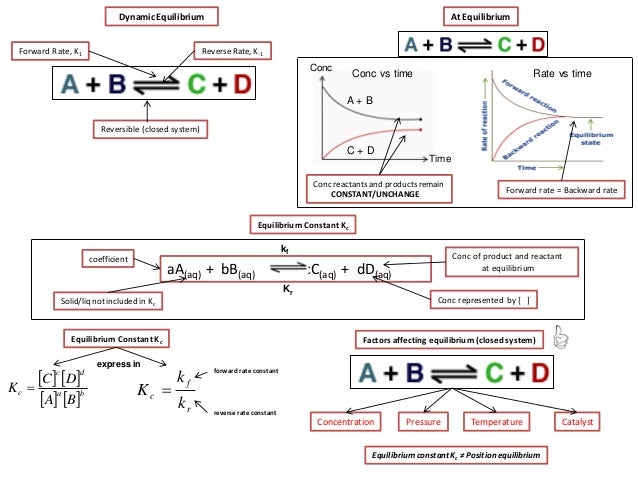
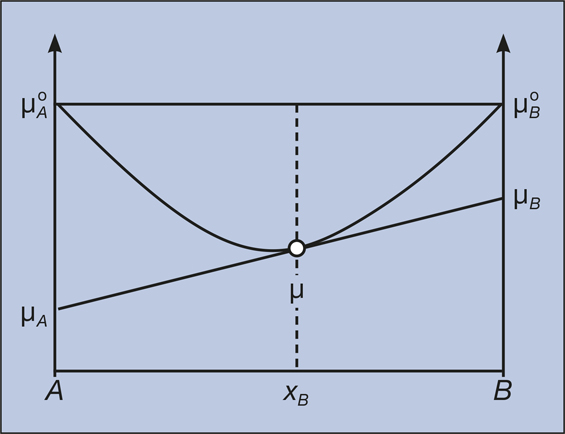
In thermodynamics the Gibbs free energy or Gibbs energy is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressureThe Gibbs free energy measured in joules in SI is the maximum amount of non-expansion work that can be extracted. In physics energy is the quantitative property that must be transferred to a body or physical system to perform work on the body or to heat it. Knowledge of free energy under one condition is compared with another allows us to predict the direction of.
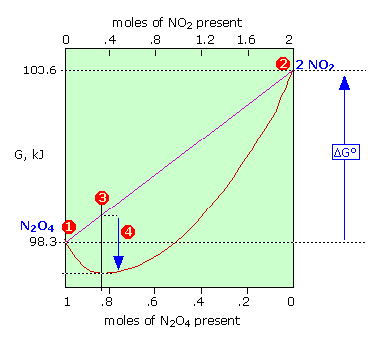
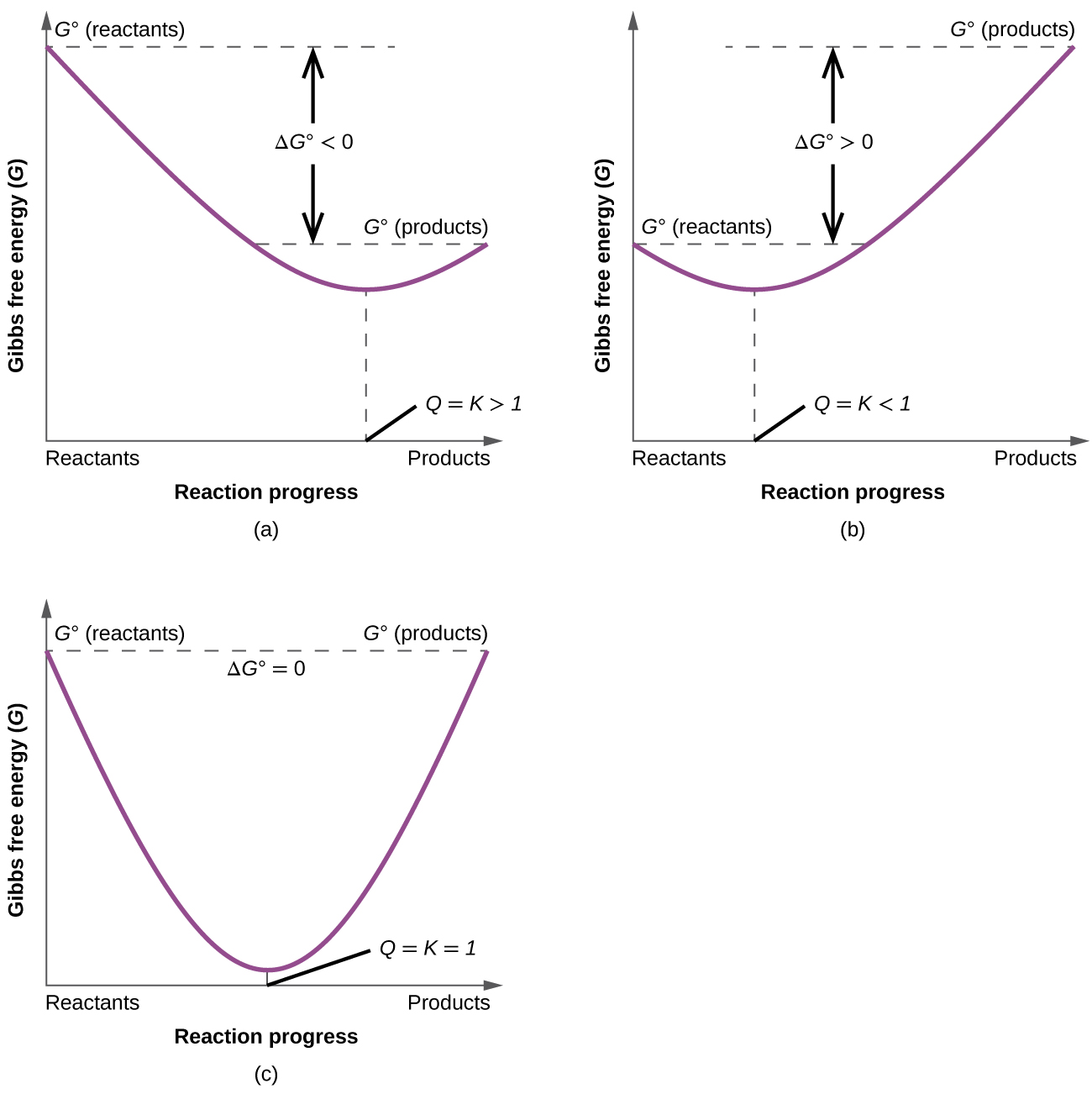
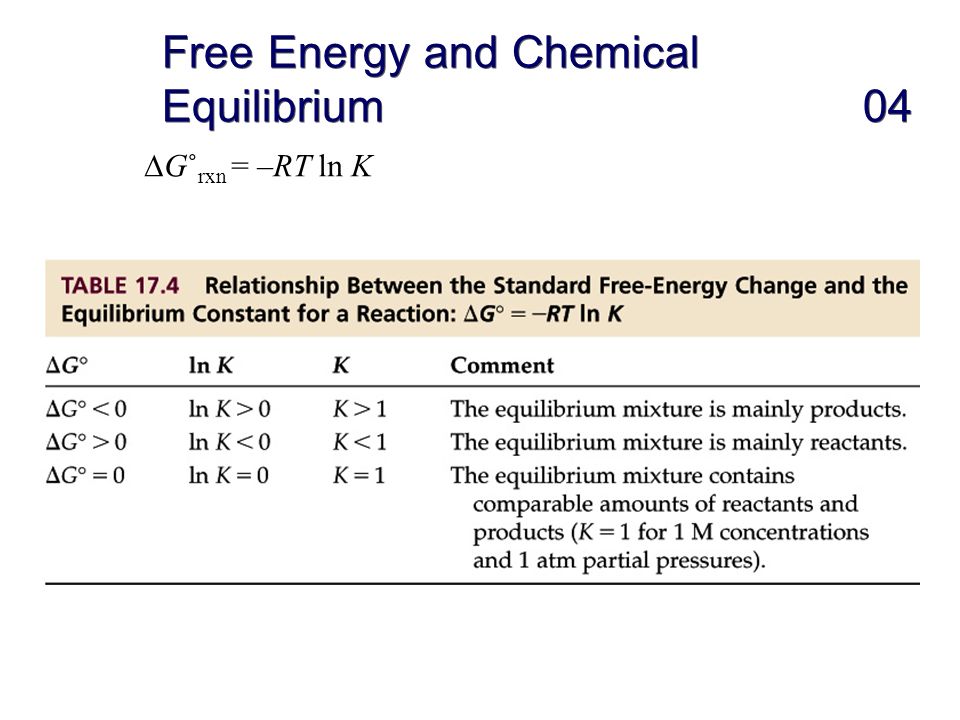
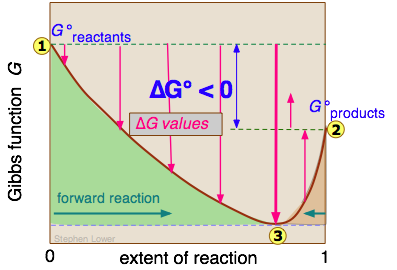
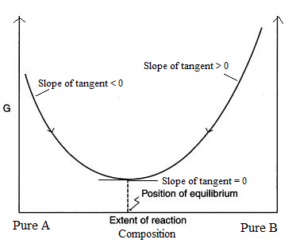
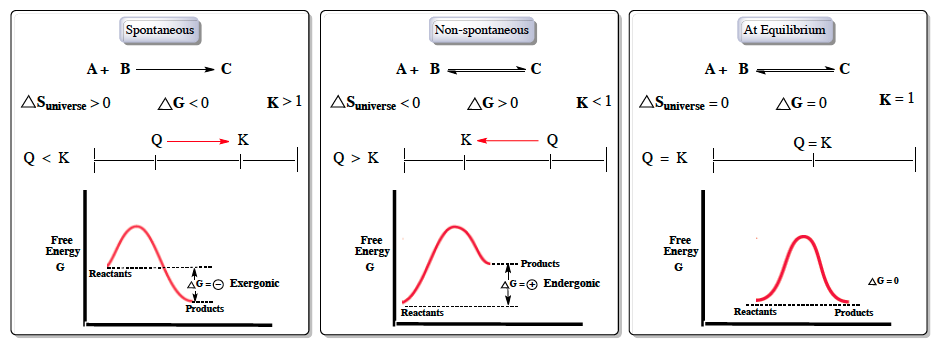
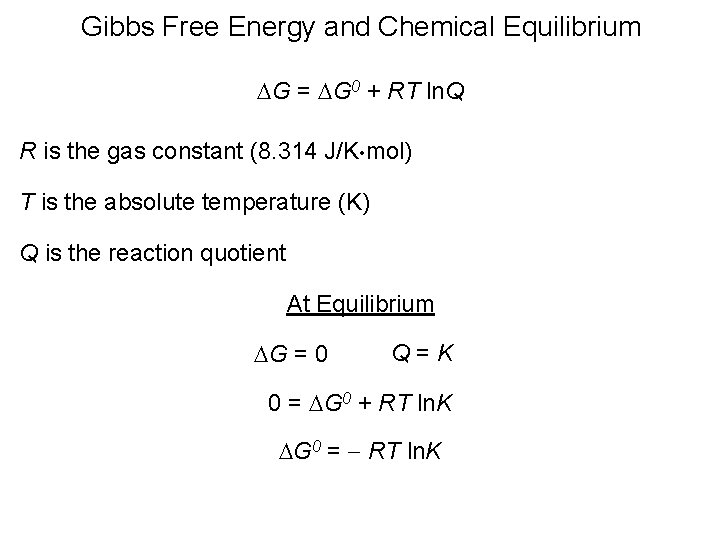
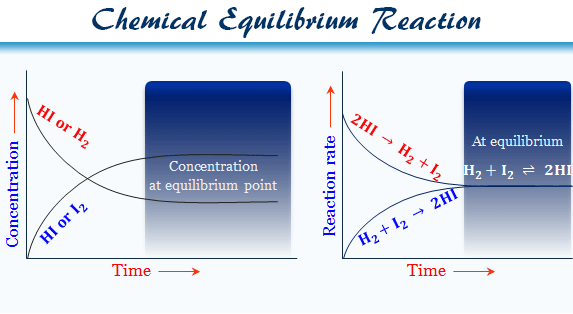
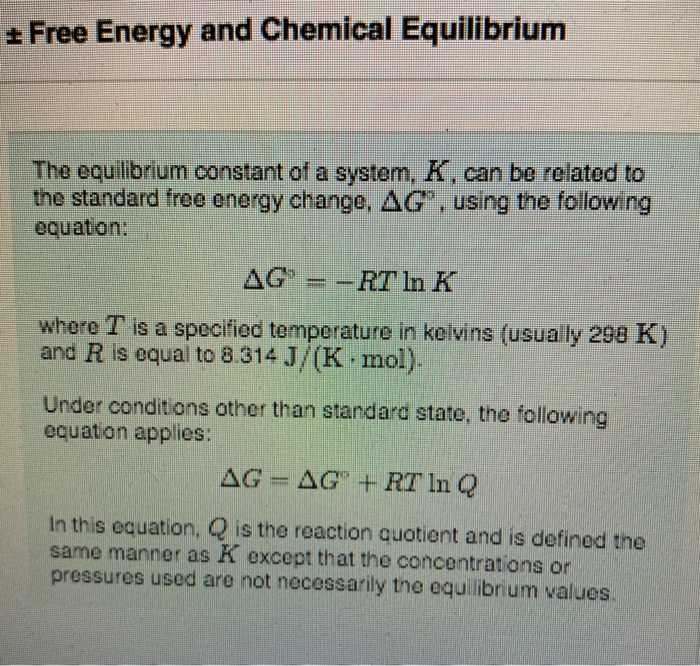
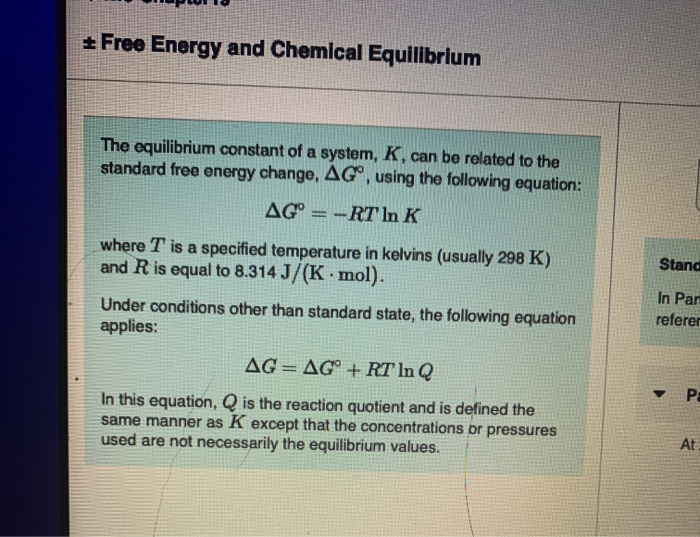
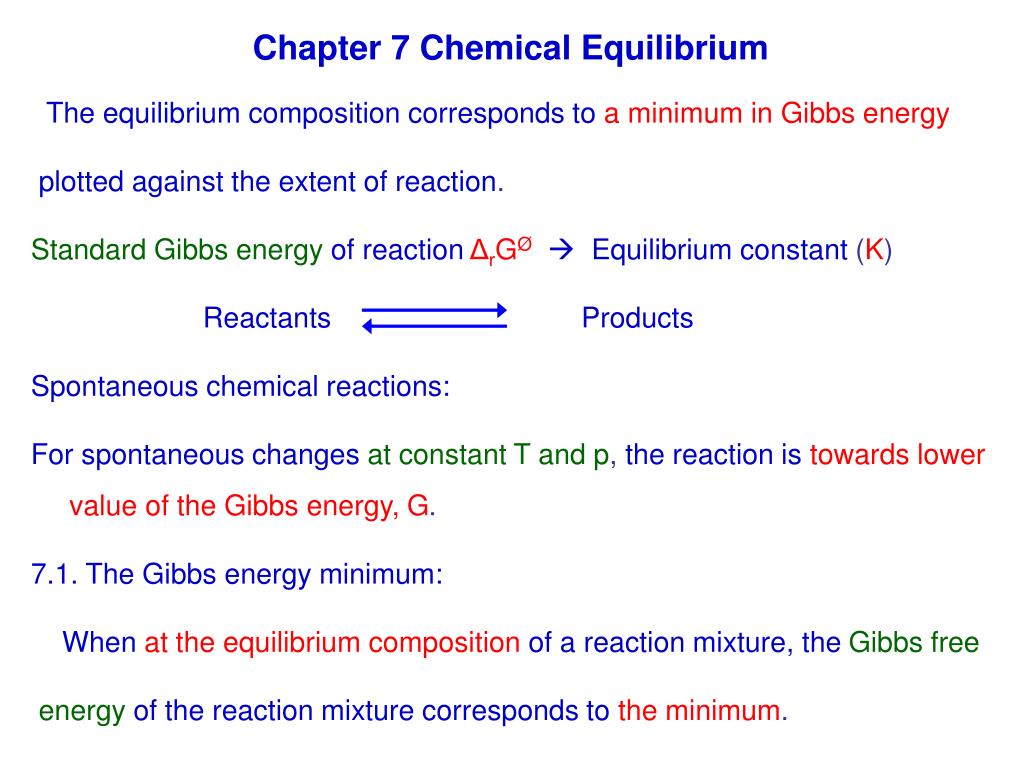
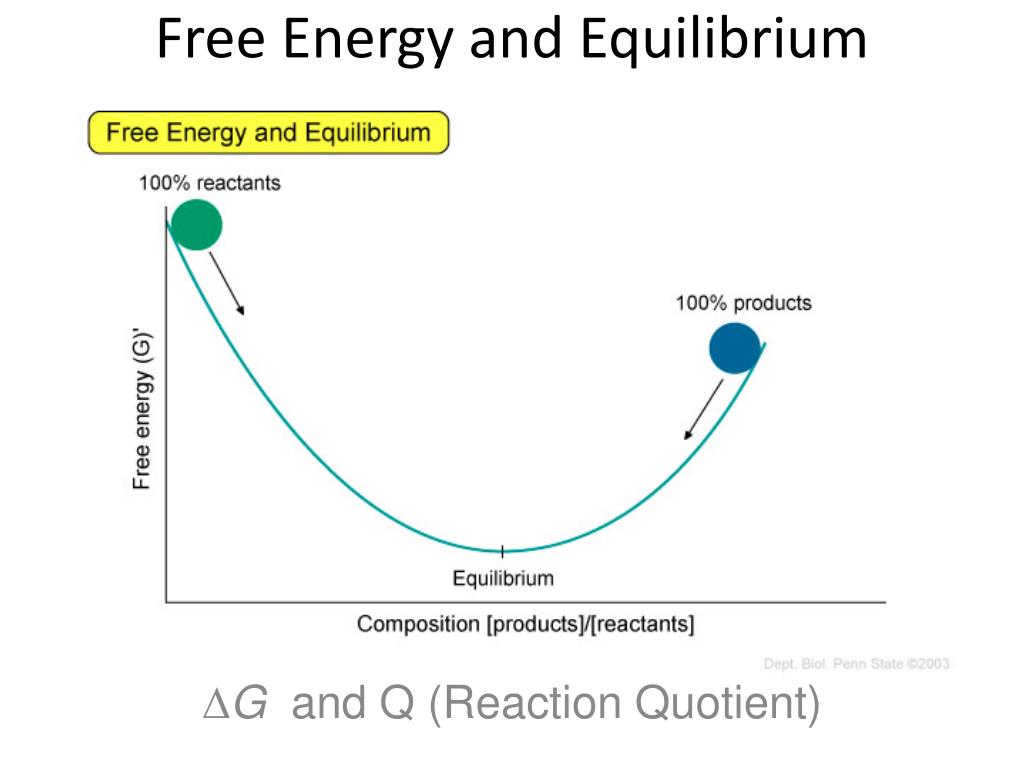
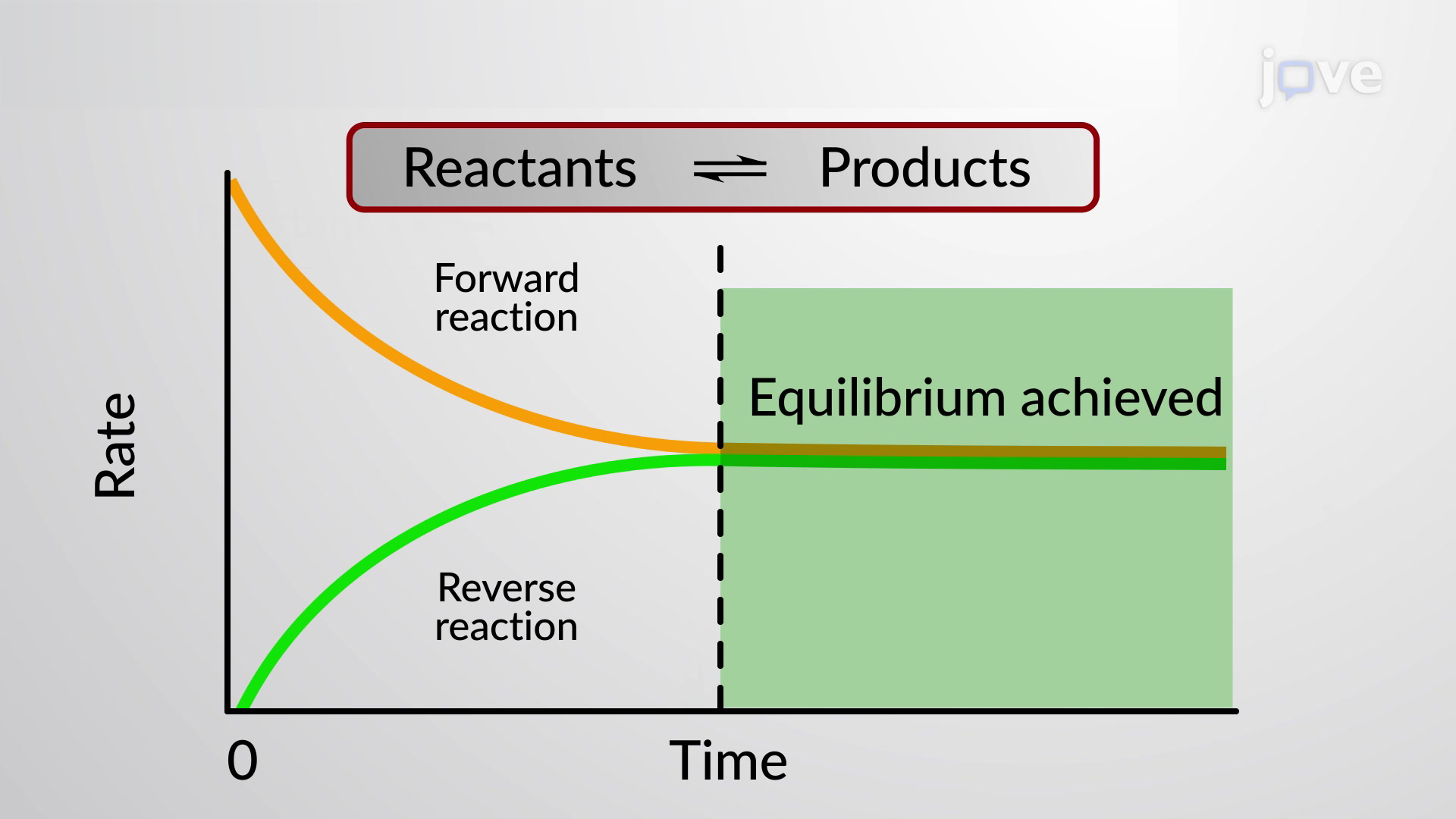
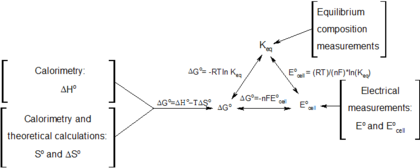
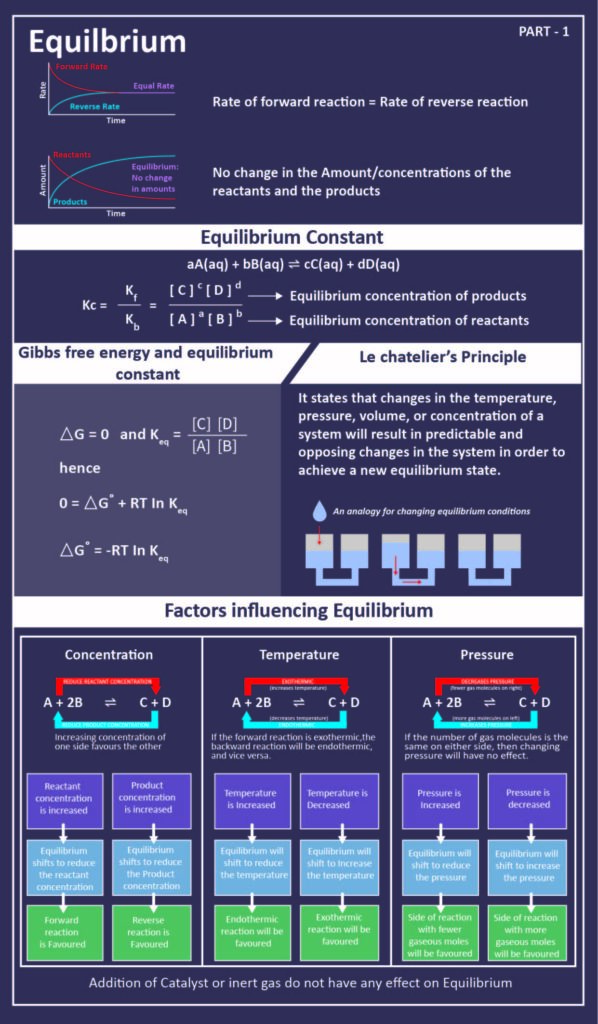
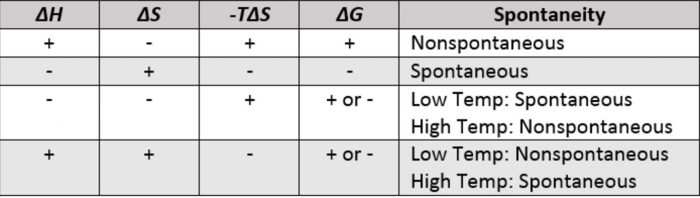
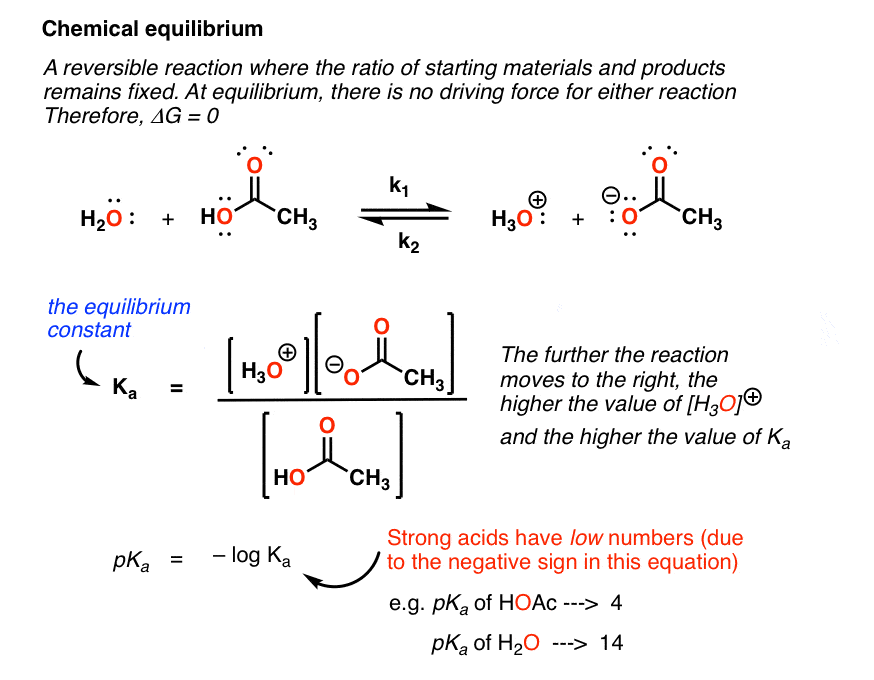
Finally the change in Gibbs free energy is zero ΔG0 for a reaction that has reached equilibrium. The law of conservation of energy states that energy can be converted in form but not created or destroyed. Likewise the change in Gibbs free energy is positive ΔG0 for a nonspontaneous process and requires the input of free energy from the surroundings.
Gibbs Free Energy and Spontaneity - Gibbs free energy is a very useful property it decreases for a spontaneous process at constant temperature and pressure. G H - T S. Energy is a conserved quantity.
In the first call to the function we only define the argument a which is a mandatory positional argumentIn the second call we define a and n in the order they are defined in the functionFinally in the third call we define a as a positional argument and n as a keyword argument. If all of the arguments are optional we can even call the function with no arguments.
In physics energy is the quantitative property that must be transferred to a body or physical system to perform work on the body or to heat it.
In thermodynamics the Gibbs free energy or Gibbs energy is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressureThe Gibbs free energy measured in joules in SI is the maximum amount of non-expansion work that can be extracted. G H - TS If the reaction is run at constant temperature this equation can be written as follows. Gibbs Free Energy and Spontaneity - Gibbs free energy is a very useful property it decreases for a spontaneous process at constant temperature and pressure. Knowledge of free energy under one condition is compared with another allows us to predict the direction of. The unit of measurement in the International System of Units SI of energy is the joule which. The law of conservation of energy states that energy can be converted in form but not created or destroyed. In the first call to the function we only define the argument a which is a mandatory positional argumentIn the second call we define a and n in the order they are defined in the functionFinally in the third call we define a as a positional argument and n as a keyword argument. Energy is a conserved quantity. Finally the change in Gibbs free energy is zero ΔG0 for a reaction that has reached equilibrium.
Energy is a conserved quantity. The unit of measurement in the International System of Units SI of energy is the joule which. Gibbs Free Energy and Spontaneity - Gibbs free energy is a very useful property it decreases for a spontaneous process at constant temperature and pressure. Knowledge of free energy under one condition is compared with another allows us to predict the direction of. In thermodynamics the Gibbs free energy or Gibbs energy is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressureThe Gibbs free energy measured in joules in SI is the maximum amount of non-expansion work that can be extracted. Likewise the change in Gibbs free energy is positive ΔG0 for a nonspontaneous process and requires the input of free energy from the surroundings. G H - T S.















Posting Komentar untuk "What Is The Change In Free Energy Of A System At Chemical Equilibrium?"